May 14, 2025
Tag:
The content of tanshinone compounds in Salvia miltiorrhiza was determined by chromatography
I. Preface
Salvia miltiorrhiza, a traditional Chinese medicine name, is the dried root and rhizome of Salvia miltiorrhiza, a plant of the Lamiaceae family and the Salvia genus.

Taking Salvia miltiorrhiza can enhance the contractility of the heart muscle, thereby improving heart function, dilating blood vessels and increasing blood flow, which is helpful in preventing and treating arteriosclerosis. Salvia miltiorrhiza itself is a traditional Chinese medicine that promotes blood circulation and removes blood stasis. It can be used to treat various blood stasis diseases. Especially when women experience dysmenorrhea, amenorrhea, and postpartum abdominal pain and other adverse symptoms, they can take Salvia miltiorrhiza for treatment. In terms of delaying aging, Salvia miltiorrhiza contains bioactive substances. On the one hand, it can enhance the activity of cells in the body; on the other hand, it can eliminate excessive free radicals in the body to prevent their peroxidation, thereby achieving the effect of delaying aging.
Tanshinone compounds are the main functional active substances of the traditional Chinese medicine Salvia miltiorrhiza and are a class of liposoluble phenquinone compounds with antibacterial effects. The 2020 edition of the Chinese Pharmacopoeia stipulates that the content of tanshinone compounds in Salvia miltiorrhiza can be determined by gas chromatography to control the quality of raw materials.
Ii. Experimental Section:
Chromatographic method for the determination of tanshinone compounds in Salvia miltiorrhiza
2.1 [Instruments and Reagents]
Instruments and equipment: GC9310-PRO Chromatograph Analytical balance (accurate to 0.0001g) Ultrasonic cleaning machines, etc.
Reagents and materials: Grade one water in compliance with GB/T6682; Methanol, acetonitrile, phosphoric acid (chromatographic grade); Tanshinone IIA reference product 0.22µm microporous nylon filter membrane.
2.2 [Experimental Method]
Preparation of reference substance solution:
Take an appropriate amount of tanshinone ⅡA reference substance, accurately weigh it, place it in a brown volumetric flask, and add methanol to prepare a solution containing 20μg per 1mL, which is the final product.
Preparation of the test solution:
Take about 0.3g of the powder of this product (passing through No.3 sieve), accurately weigh it, place it in a conical flask with a stopper, accurately add 50mL of methanol, tightly stopper, weigh it, ultrasonically treat it (power 140W, frequency 42kHz) for 30 minutes, let it cool, weigh it again, make up for the lost weight with methyl alcohol, shake well, filter, and take the filtrate, which is the product.
2.3 [Chromatographic Conditions]
a) Chromatographic column: C18 4.6×250mm, 5μm chromatographic column
b) Mobile phase A: Acetonitrile; Mobile phase B: 0.02% phosphoric acid solution; The gradient program elution is as follows:
Table 1 Gradient Elution Table
| Time (min) | Mobile phase A | Mobile phase B |
|
0 |
61 |
39 |
|
6 |
61 |
39 |
|
20 |
90 |
10 |
|
20.5 |
61 |
39 |
|
35 |
61 |
39 |
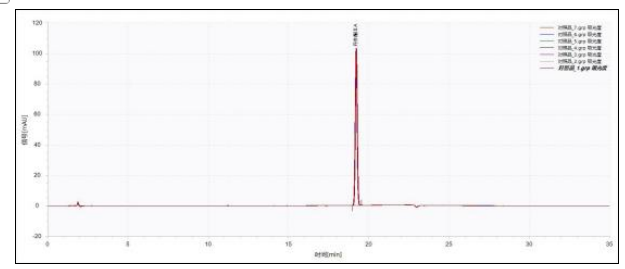
Figure 1 Chromatogram of Tanshinone IIA reference solution (n=7)
The chromatogram of the test solution is shown in Figure 2. Taking tanshinone ⅡA as the reference and its corresponding peak as the S peak, the relative retention times of cryptotanshinone and tanshinone I were calculated. The relative retention times of both were within ±5% of the specified values. Seven consecutive injections were used for repeatability testing. The RSD values of retention time and peak area for tanshinone ⅡA, cryptotanshinone, and tanshinone I are calculated as shown in Table 2.
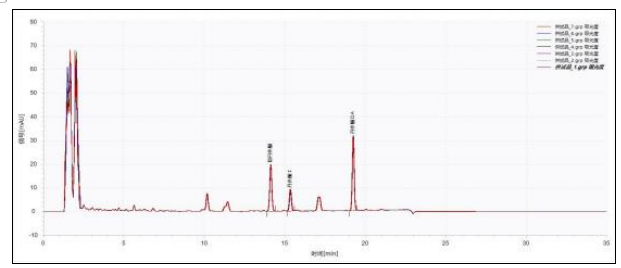
Table 2 Statistics of repeatability Data of the test solution
| Compound | Average value |
SD |
RSD% |
|
|
Tanshinone ⅡA |
19.265 |
0.013 |
0.067 |
| Retention time | Yintanshinone |
14. 142 |
0.012 |
0.087 |
|
|
Tanshinone I |
15.363 |
0.016 |
0.105 |
|
|
Tanshinone ⅡA |
264.670 |
0.718 |
0.271 |
| Peak area | Yintanshinone |
178.927 |
0.570 |
0.319 |
|
|
Tanshinone I |
78.412 |
0.409 |
0.522 |
[Conclusion]
The experimental results show that when using the GC9310-PRO chromatograph to test the tanshinone compounds in Salvia miltiorrhiza, the peak areas of tanshinone ⅡA, cryptanshinone, and tanshinone I in the reference solution and the test sample have good reproducibility, and the RSD values of the peak areas are all less than 0.550% (n=7). The theoretical plate number of tanshinone ⅡA in the test sample reached 120,811.210, fully meeting the requirement of no less than 60,000 stipulated in the 2020 edition of the Chinese Pharmacopoeia. This method can be used for tanshinone compounds in Salvia miltiorrhiza


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


